ALS Research at UMass Chan Medical School
The Angel Fund for ALS Research supports the ALS (Lou Gehrig’s Disease) research at UMass Chan Medical School in Worcester, MA. The lab is under the direction of Dr. Robert H. Brown, Jr.
You can read about the Brown Lab:
Dept of Neurology at UMass Medical School
A Research Update – July 1, 2024
by Daryl Bosco, PhD
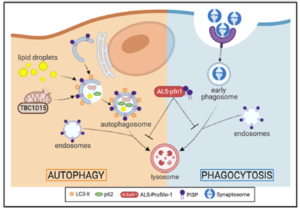
 With support from the Angel Fund, the Bosco laboratory recently completed a study that examined how a specific type of immune cell becomes dysfunctional in ALS. In addition to motor neurons, which ultimately degenerate in ALS, there are other cell types in the central nervous system (CNS) that can influence the course of ALS. For example, microglia are immune cells that become alert to injury and infection, and in doing so, help to protect the CNS. However, in ALS and related disorders, microglia become abnormal and lose their ability to protect the CNS and instead become toxic to neurons. To study microglia in ALS, the Bosco laboratory is using a state-of-the-art cell culture model system that involves induced pluripotent stem cells, which can be transformed into human microglia as well as neurons and other CNS cell types. A major advantage of this system is it allows researchers to study human CNS cells, as opposed cells from other species, which may not always be relevant to human diseases such as ALS. The Bosco laboratory discovered that microglia expressing the ALS-linked gene profilin-1 are defective in their normal, protective function of phagocytosing (or ingesting) debris in the CNS, which in turn leads to an unhealthy CNS. Their reports showed that treating ALS microglia with rapamycin rescued this defect, raising the possibility that this pathway could be targeted therapeutically in ALS. Currently, the Bosco laboratory is examining human microglia with other ALS-linked genes, including TDP-43, which is associated with >97% of ALS cases. The Bosco laboratory is also studying human motor neurons to identify mechanisms of degeneration that could be inhibited as a therapeutic intervention for sporadic ALS.
With support from the Angel Fund, the Bosco laboratory recently completed a study that examined how a specific type of immune cell becomes dysfunctional in ALS. In addition to motor neurons, which ultimately degenerate in ALS, there are other cell types in the central nervous system (CNS) that can influence the course of ALS. For example, microglia are immune cells that become alert to injury and infection, and in doing so, help to protect the CNS. However, in ALS and related disorders, microglia become abnormal and lose their ability to protect the CNS and instead become toxic to neurons. To study microglia in ALS, the Bosco laboratory is using a state-of-the-art cell culture model system that involves induced pluripotent stem cells, which can be transformed into human microglia as well as neurons and other CNS cell types. A major advantage of this system is it allows researchers to study human CNS cells, as opposed cells from other species, which may not always be relevant to human diseases such as ALS. The Bosco laboratory discovered that microglia expressing the ALS-linked gene profilin-1 are defective in their normal, protective function of phagocytosing (or ingesting) debris in the CNS, which in turn leads to an unhealthy CNS. Their reports showed that treating ALS microglia with rapamycin rescued this defect, raising the possibility that this pathway could be targeted therapeutically in ALS. Currently, the Bosco laboratory is examining human microglia with other ALS-linked genes, including TDP-43, which is associated with >97% of ALS cases. The Bosco laboratory is also studying human motor neurons to identify mechanisms of degeneration that could be inhibited as a therapeutic intervention for sporadic ALS.
This is a link to the report:
https://pubmed.ncbi.nlm.nih.gov/38509062/
research update – December 2022
research update – August 1, 2022

